News
Fill & Finish
Best in class service for batches ranging from 100 to 3,000 vials. Our solution-driven GMP-compliant processes come with unparalleled flexibility, fully-adaptable to the unique needs of our clients.
Our scientists are highly experienced in keeping product losses and vial rejections to an absolute minimum, which is especially critical for clients with low bulk volumes (1– 10 L) to avoid losses of precious product.
We offer the complete spectrum of Fill & Finish services under one roof – from aseptic filling to QC tests, labeling, release, storage and shipping.
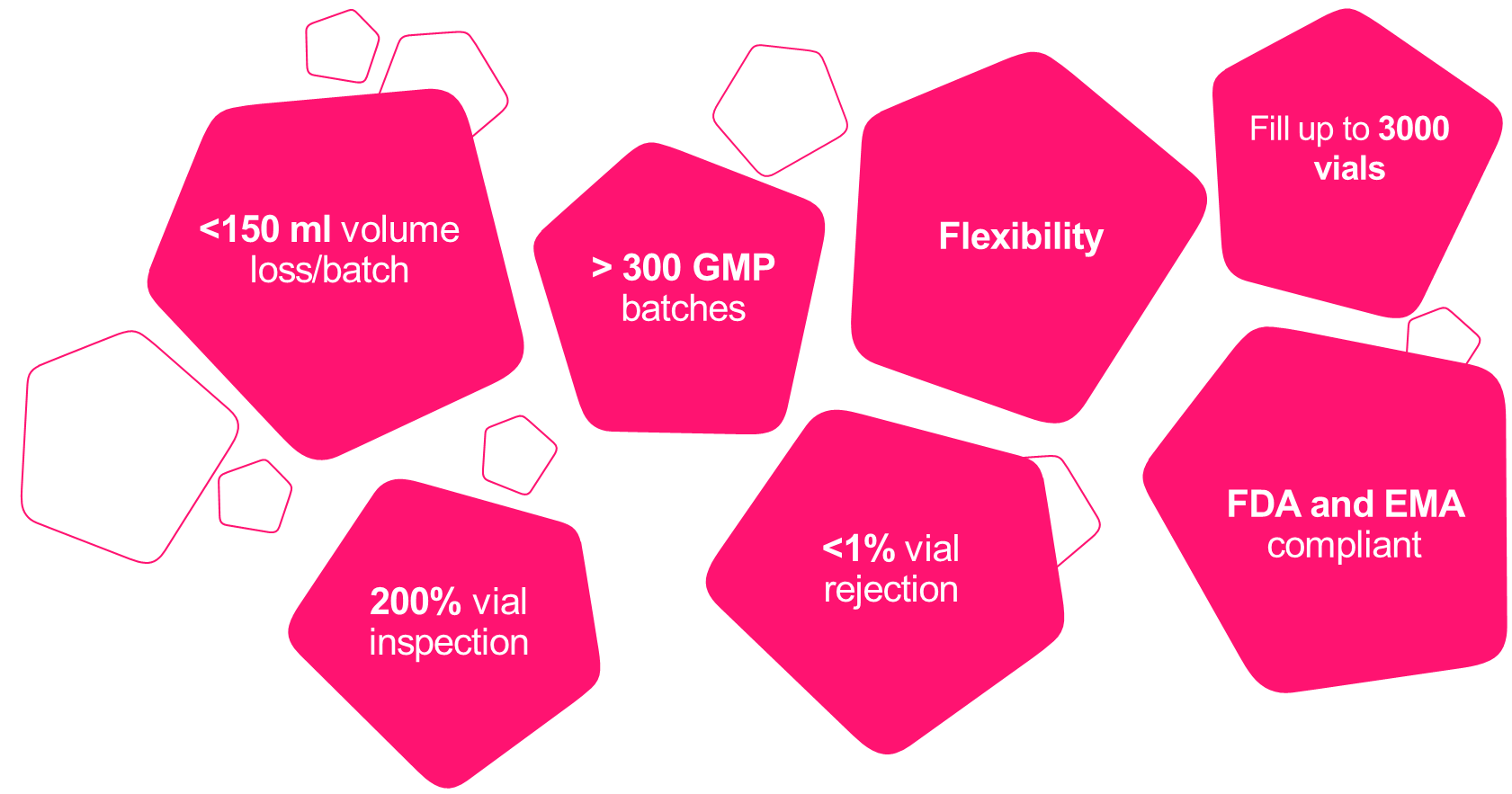
Summary of technical specifications:
- Container:
- Vial
- Formulation:
- Liquid
- Vials validated:
- 2-50R
- Fill Volume:
- 0.3–51 mL
- Vial Capacity:
- up to 3000 vials
- Machineries:
- 4 different semiautomatic machines from which the customer can choose the most suitable for his specific needs
- Product expertise:
- Monoclonal Antibodies, Recombinant Proteins, and Vaccines
- Regulatory compliance:
- FDA and EMA
- One Stop service:
- Aseptic Filling, QC Methods, Labelling, Release, Storage and Shipping.
- Storage room temperatures:
- From ambient to –80°C