Capabilities & Facilities
At our GMP facilities in Heidelberg and Basel, we offer production of drug substances and drug products for biopharmaceutical applications. The fermentation technologies we apply comprise both fed-batch and perfusion in single-use or stainless steel bioreactors.

Site Heidelberg
At our Heidelberg site, we operate facilities comprising 1250 sqm clean rooms of clean room class B, C or D for GMP production and quality control.
Clean room area: 1250 sqm
Liquid handling capacity: up to 100,000 L per run
For our 5 GMP lines we offer capacities of:
- 10 L single-use
- 50 L single-use
- 100 L stainless steel
- 200 L single-use
- 1000 L single-use
- 2000 L single use
with corresponding downstream process equipment.
Site Basel
At our Basel site, we operate facilities comprising 500 sqm clean rooms of clean room class C for GMP production, as well as labs for cell line development, process development and quality control.
Lab and Production area: 950 sqm
Liquid handling capacity: up to 2000 L per run
On our 2 GMP lines we offer capacities of:
- 50 L GMP, single use
- 200 L GMP, single use
- 250 L GMP, single use
- 1000 L GMP, single use
with corresponding downstream process equipment.


Fill & Finish
Our scientists are highly experienced in keeping product losses and vial rejections to an absolute minimum, which is especially critical for clients with low bulk volumes (1– 10 L) to avoid losses of precious product.
We offer the complete spectrum of Fill & Finish services under one roof – from aseptic filling to QC tests, labeling, release, storage and shipping.
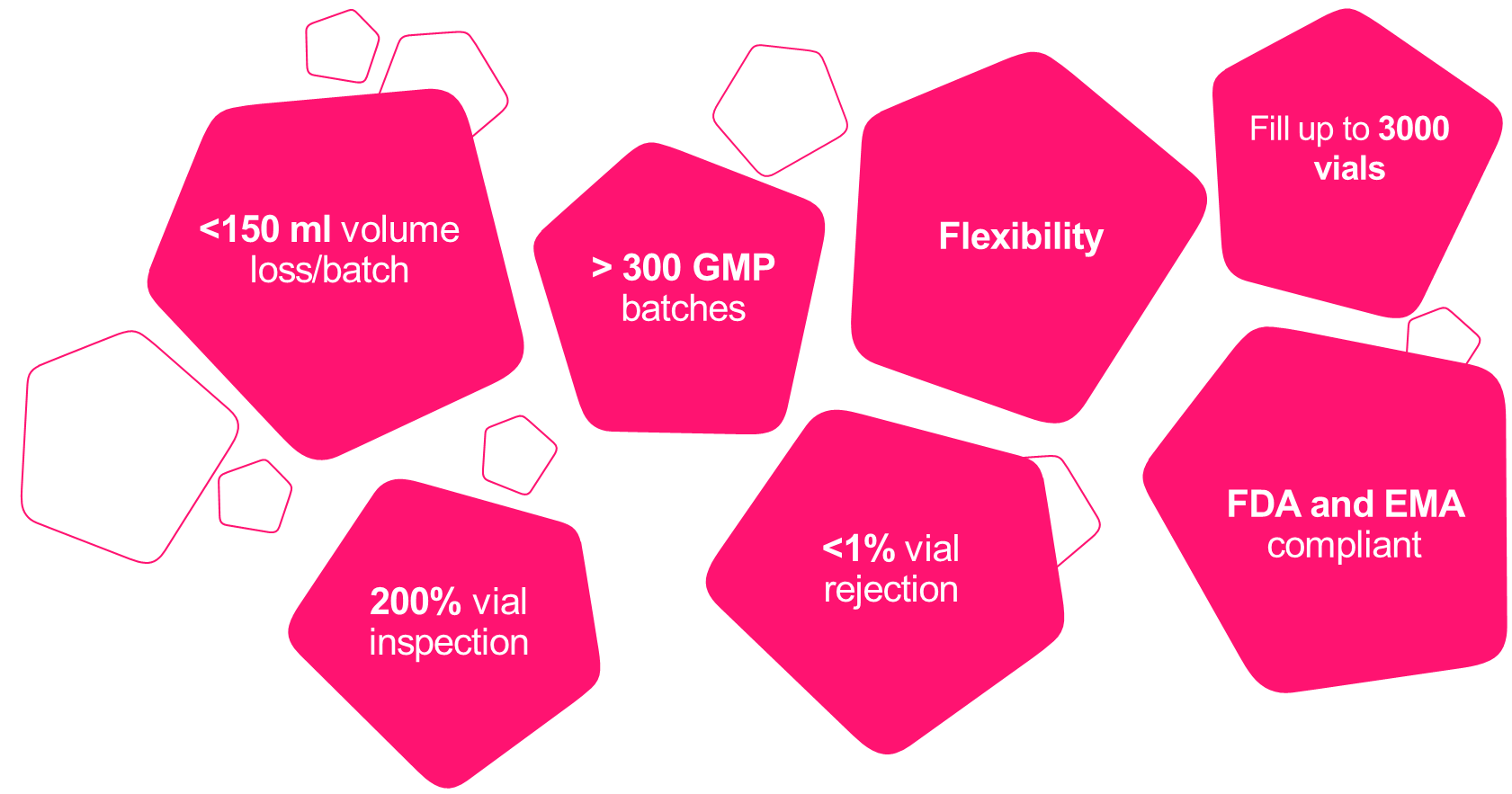
Summary of technical specifications:
- Container:
- Vial
- Formulation:
- Liquid
- Vials validated:
- 2-50R
- Fill Volume:
- 0.3–51 mL
- Vial Capacity:
- up to 3000 vials
- Machineries:
- 4 different semiautomatic machines from which the customer can choose the most suitable for his specific needs
- Product expertise:
- Monoclonal Antibodies, Recombinant Proteins, and Vaccines
- Regulatory compliance:
- FDA and EMA
- One Stop service:
- Aseptic Filling, QC Methods, Labelling, Release, Storage and Shipping.
- Storage room temperatures:
- From ambient to –80°C




